Introduction
Relapsed/refractory multiple myeloma (RRMM) is a hematologic cancer associated with no current widely-accepted cure, cycles of relapse and remission, treatment resistance, and high morbidity and mortality, which necessitate new treatments and therapeutic targets. 1-3 Innovative therapies that target BCMA, CD38, GPRC5D, FcRH5, and others in the RRMM development pipeline are recently approved or under investigation, but real-world evidence on clinician perceptions of emerging trends and unmet needs remains limited. A Delphi panel was conducted with US clinicians to evaluate the level of unmet need and treatment decisions among patients with RRMM who are triple-class-exposed (TCE).
Methods
This double-blinded study consisted of two phases: a web survey (phase 1) and a Delphi panel (phase 2). A targeted literature review was conducted first to understand unmet needs and treatment decisions associated with management of RRMM in the US and inform the development of primary research materials. The web survey was completed by 21 US clinicians. Survey findings were then used to inform the phase 2 Delphi panel, a systematic, validated approach to gain consensus. This consisted of two iterative rounds of a question answered by nine experts selected from participants in the web survey. Consensus on a question was defined as at least 70% agreement. The double-blinded panel was conducted via the Microsoft Teams platform, and poll questions were administered through Vevox. Responses were blinded to preserve respondents' anonymity and participants were also blinded to the study sponsor.
Results
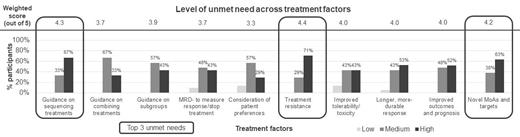
Overall, consensus was reached that despite recent improvement in treatment landscape, there is still a high level of unmet need for patients with RRMM who are TCE (89% of participants). More specifically, consensus was reached (89%) that the top three areas of unmet need were treatment resistance, guidance on treatment sequencing, and novel mechanisms of action (MoAs) and targets (Figure 1). Unmet need is also particularly pronounced for certain patient subgroups, with 89% and 78% of participants agreeing that patients who are functionally high-risk and penta-refractory, respectively, experience the greatest unmet need. When asked about which novel therapies would be most promising for addressing the unmet needs, participants agreed that the top 4 therapeutic targets are GPCR5D (95%), BCMA (81%), FcRH5 (81%), and BCL-2 (81%).
Various factors impact treatment choice and sequencing, with most participants (78%) agreeing that treatment history is the most influential factor impacting sequencing. The panel also agreed that prior toxicity (grade 3-4 AEs, 89%) and convenience of dosing frequency (78%), respectively, most impact dosing decisions. All agreed there is a need for resources to manage some AEs, particularly newer AEs (like skin and nail effects) of emerging treatments. Of the AEs identified in the survey as top drivers of treatment choice, participants reached consensus on two: neurotoxicity (e.g., ICANS) (89%) and infection (100%).
Conclusions
In this Delphi study, consensus was reached that a high level of unmet need remains for patients with RRMM, who are TCE with unmet need varying dependent on patient subgroup and treatment history. There was consensus that additional treatment choices with novel targets/MoAs are needed. Participants agreed that AEs, toxicity, infection, and treatment history impact treatment choice and sequencing, and considerations and challenges vary by subgroup. Overall, findings from this study support that there is a need for additional treatment options with novel MoAs/therapeutic targets that address priority unmet needs for patients with RRMM, who are TCE.
References
1. Gonsalves W et al. The next generation of novel therapies for the management of relapsed multiple myeloma. Future Oncol (London, England). 2017;13(1):63-75. https://doi.org/10.2217/fon-2016-0200
2. Braunstein M et al. A new decade: novel immunotherapies on the horizon for relapsed/refractory multiple myeloma. Expert Rev Hematol. 2022;14(4):377-389. doi:10.1080/17474086.2021.1909469
3. Bruno AS et al. Recent real-world treatment patterns and outcomes in US patients with relapsed/refractory multiple myeloma. Expert Rev Hematol. 2020;13(9):1017-1025. doi:10.1080/17474086.2020.1800451
Disclosures
Fonseca:Antegene: Membership on an entity's Board of Directors or advisory committees; AZBio: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Sanofi: Consultancy; Regeneron: Consultancy; Janssen: Consultancy; Millenium: Consultancy; Pfizer: Consultancy; Caris Life Sciences: Membership on an entity's Board of Directors or advisory committees; FISH: Patents & Royalties: FISH; Pharmacyclics: Consultancy; Merck: Consultancy; Juno: Consultancy; Kite: Consultancy; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; BMS (Celgene): Consultancy; Binding Site: Consultancy; Bayer: Consultancy; Aztrazenica: Consultancy; AMGEN: Consultancy; Adaptive Biotechnologies: Consultancy; AbbVie: Consultancy. Le:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Lodowski:PRMA Consulting Limited: Current Employment; Janssen: Other: PRMA Consulting Ltd was contracted by Janssen to work on this project. DeBrosse:PRMA Consulting Limited: Current Employment, Other: PRMA Consulting Ltd was contracted by Janssen. Daniel:PRMA Consulting Limited: Current Employment, Other: PRMA Consulting Ltd was contracted by Janssen. Narvekar:PRMA Consulting Limited: Current Employment, Other: PRMA Consulting Ltd was contracted by Janssen. Zhang:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal